CIBMTR Corporate Studies and Registries Forms Due List
The Corporate Studies and Registries Forms Due List is automatically sent each Tuesday via email to current Primary Data Managers and staff we’ve been asked to copy on correspondences sent to the primary data manager role. If you have questions about forms in these reports, contact CIBMTR Center Support.
If your center is not receiving this report, your primary data manager may submit a CIBMTR Center Support ticket to request assistance with the distribution list including adding staff members to the distribution list.
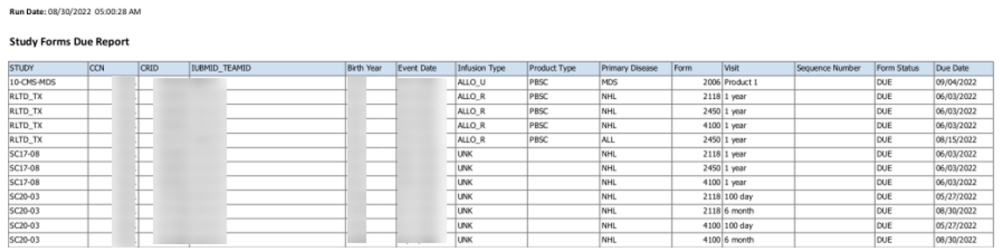
Your center will receive this list if there are any forms DUE for any study. The table will contain one row per form due for the studies that your center is participating in. Forms will be listed if the due date is past or within seven days of the run date.
These forms are a subset of forms due for CPI. Completing these forms by the form due date will lower the forms due in the next CPI trimester. The form due date is the date by which CIBMTR expects to receive the data whenever possible. Forms for a study are expected to be completed by the form due date to ensure all required data are available for analysis.
Definitions
Run Date
Indicates the date and time the report was generated. Times listed are US central time, not necessarily the center’s local time. There may be a delay between the report generation and when it is distributed to transplant centers. Use the Center Forms Due feature in FormsNet for a real-time listing of what forms are outstanding.
Study Name
Indicates the CIBMTR study number. There will be one row per form for that study. A study may have multiple rows for as many forms as there are due.
Recipient details
CRID, IUBMID/Team ID, birth year and event date are all provided to help identify the correct recipient.
Infusion details
There is a column for Infusion Type, Product type, and Primary Disease.
Form details
Form ID, visit, sequence number, form status and due date are provided for each form. The table will contain any form past due and due within 7 days of the run date.

