Compliance Monitoring
Centers should monitor their compliance to the CPI requirements throughout the trimester.
The form completion categories are important to monitor as the total number of forms needed to meet the targets will fluctuate throughout the trimester. This fluctuation is a result of forms coming due after more data are provided. Examples include:
- Registering a new CRID will result in an Indication for CRID Assignment (2814) Form to come due. If an HCT is reported on the F2814, then a Pre-TED (2400) Form and Disease Classification (2402) Form will come due.
- When completing a CRF Post-Infusion Follow-Up (2100) Form, reporting a particular fungal or viral infection will result in the associated disease insert form to come due.
If the due date for any additional forms falls within the applicable trimester, the form will count toward the total forms expected for that trimester.
Monitoring Tools
Centers can monitor their compliance to each CPI requirement by using the CPI Summary report. For specific forms due in the assessment trimester, centers can use the CPI Summary reports & utilize the Center Forms Due tool within FormsNet3.
CPI Compliance Stages
A center is in a Good Standing status if all CPI requirements are met by the end of the trimester.
If a center ends the trimester in a non-compliant status, one of the non-compliance statuses, as defined below, are assigned. The center’s Primary Data Contact and Medical Director will receive a notification from the CIBMTR detailing the specific unmet CPI standards, the status assigned, and additional details as described below.
- First Warning: First trimester of non-compliance
- Probation: Second consecutive trimester of non-compliance
- Suspension: Third consecutive trimester of non-compliance
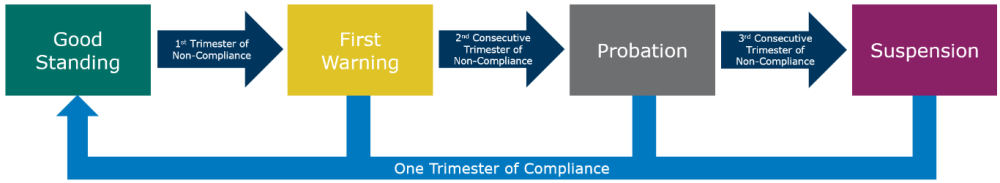
If the center does not return to a Good Standing status (must meet all target completion metrics) after the first trimester of non-compliance (First Warning), the center will move to the next stage of non-compliance (Probation). At this point, CIBMTR will contact the center to schedule a meeting to discuss the center’s backlog. These meetings require attendance of the center’s Medical Director and CIBMTR’s Scientific Director. The goal of this meeting is to review the forms backlog and develop a comprehensive corrective action plan that addresses the outstanding forms and includes plans for ensuring the center stays in a Good Standing status once achieved again. This may result in the creation of a CPI Exemption plan, which will outline specific milestones the center must achieve in order to avoid Suspension. Additionally, CIBMTR study teams will be notified of the center’s non-compliance and may take action to reduce or terminate a centers participation in ongoing or new studies.
If the center is non-compliant for a third consecutive trimester, the center will move to Suspension status. At this point, CIBMTR leadership will determine what additional consequences will be applied based on the forms backlog and quality concerns. These consequences may include:
- Exclusion from the annual Transplant Center Specific Survival Analysis (TCSA)
- Exclusion from Observational Research Studies
- Change to TED-only reporting (see Determining Reporting Levels)
- Deny center CIBMTR leadership roles or membership in CIBMTR administrative committees
- Restrict access to NMDP unrelated donors

