The Indication for CIBMTR Data Reporting (2814) Form collects information to initiate CIBMTR reporting on the appropriate research or data collection forms. This form must be completed for the first indication requiring the individual to register for a CIBMTR Research ID (CRID) and any subsequent infusions the recipient received.
- First infusions: This is the first form completed after the CIBMTR Research ID Assignment (2804) Form.
- Subsequent infusions: This form is triggered from the Post-Transplant Essential Data (2450), Post-HCT Follow-up Data (2100), or Post-Cellular Therapy Essential Data (4100) forms when a subsequent infusion is reported.
On-Demand reporting
There are two appropriate scenarios where centers should create an on-demand Form 2814:
1. To report a subsequent infusion when there are NO follow up forms (F2100, F2450 or F4100) available to report this information.
2. To report DLIs “on time”
For steps on how to create an on-demand form, see ‘Create an Unscheduled Form’ within the FormsNet3SM Training Guide.
The combination of answers provided in this form is used to determine the infusion type and which forms are required. Refer to Tables 1 and 2 for reference.
Event date
If the form is in DUE status, the event date displayed in the Recipient Forms Grid will be the date the form was created. When the form is submitted, the event date will update to the infusion date provided on the form (for HCT, gene therapy, or cellular therapy) or it will be updated to the appropriate allogeneic HCT event date for DLIs.
Determination of Infusion Type and Forms Required
The combination of answers provided will determine the infusion type (HCT, gene therapy, cellular therapy or DLI). Review the tables below to understand how the infusion type is determined, and which forms are required.
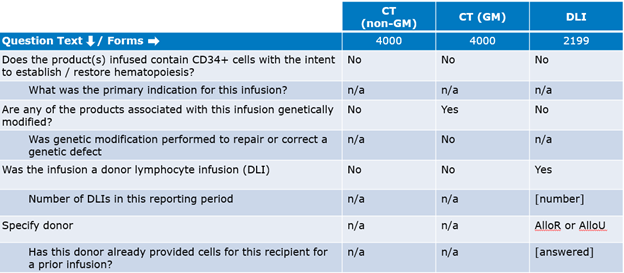
Table 1. Reporting HCTs and Gene Therapies

Table 2. Reporting Cell Therapies
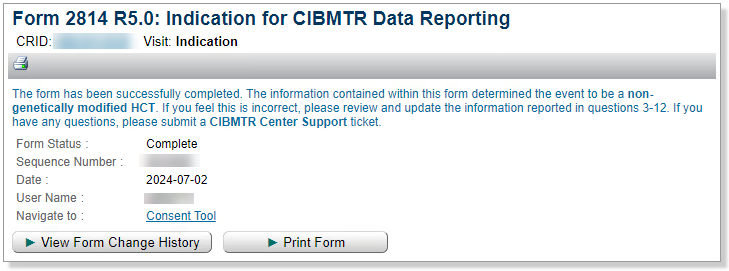
Form Submission Message
When the Form 2814 is submitted, a message will display showing the infusion type.
The infusion type will also display in the Recipient Forms Grid in the Visit Details column.

Editing the F2814
This form can be edited until the Pre-TED (2400) and Disease Classification (2402) Forms or Pre-CTED (4000) Form is completed and submitted.
If the infusion type displayed on the form completion page or in the Visit Details column of the Recipient forms grid is incorrect, go back and edit the form. Refer to Table 1 and 2 to understand how the infusion type is determined
If any changes to the form need to be made after these forms are submitted, contact CIBMTR Center Support .
Section Updates:
| Question Number | Date of Change | Add/Remove/Modify | Description | Reasoning (If applicable) |
|---|---|---|---|---|
| . | . | . | . | . |
Need more help with this?
Don’t hesitate to contact us here.

