Question 41: Specify the timepoint in the product preparation phase that the product was analyzed
For all products, the “at infusion” timepoint must be reported. The “at infusion” timepoint should only report the values for the actual product volume infused.
The At Infusion timepoint should include values reflective of the product infused regardless of when the analysis occurred. Since all products are analyzed prior to cryopreservation, the At Infusion timepoint would be applicable for these cell counts. Depending on the product type and your center’s practice, viability may be assessed closer to the time of infusion.
For cord blood units, an “At Arrival” timepoint should be reported if the center performed an analysis prior to the CBU wash. An “At Infusion” timepoint must be reported and should reflect the product analysis performed post wash.
Cord Blood Units: Centers are reminded to only report product testing performed by their laboratory. Product testing performed by the cord blood bank is captured in the Product Transport and Receipt section of this form and should not be reported in the Product Analysis section. If the transplant center only tests for viability, report the timepoint, date of analysis, product volume, and viability.
Question 42: Date of product analysis
Report the date the product was analyzed. For the “At Infusion” timepoint, if the product was analyzed multiple times after arriving at the transplant center, report the latest date the product was analyzed with the associated cell counts prior to infusion. The date of product analysis is not necessarily the date of the product infusion.
If a product is analyzed multiple times prior to product infusion, the type of product will determine which analysis to report for the At Infusion timepoint. See below for more information:
Fresh product: If an unmanipulated, fresh product was analyzed multiple times prior to infusion, the most recent complete analysis should be reported for the At Infusion timepoint.
Example 1: Upon receiving a fresh product, the transplant center completes a TNC, CD34, and viability analysis. The product was not manipulated but prior to infusion, a small sample was collected to analyze the viability. The analysis performed upon receiving the fresh product should be reported for the At Infusion timepoint.
Cryopreserved product: If a cryopreserved product is infused, report the complete analysis, adjusted for the volume infused, performed upon either at arrival of the product or prior to cryopreservation for the At Infusion timepoint. If the cryopreserved product is contained in multiple bags, only report the sum of the cell counts for the bags infused. If the cryopreserved product is contained in a single bag, report the cell counts adjusted for the volume infused. In the rare scenario where a complete analysis performed post-thaw, this analysis should be reported for the At Infusion timepoint; however, this is unlikely as there is usually not enough product to perform a complete analysis post-thaw.
Example 2: Upon collecting an autologous PBSC product, the transplant center completes a TNC, CD34, and viability analysis. The product is separated into three bags and cryopreserved. Two of the three bags were thawed, the TNC and viability were analyzed, and the product was infused. The analysis performed upon collecting the product, adjusted for the two bags infused (the sum of volume and cell counts) should be reported for the At Infusion timepoint.
Processed product: Report the last analysis performed prior to product infusion for the At Infusion timepoint.
Example 3: Upon receiving a PBSC product, the transplant center completes a TNC, CD34, and viability analysis and then RBC reduced the product. After processing, the CD34 and viability are analyzed. The analysis performed after RBC reduction (CD34 and viability) should be reported for the At Infusion timepoint. In this scenario, the analysis for the TNC performed prior to RBC reduction will not be reported.
Question 43: Total volume of product plus additives
Enter the total volume of the product plus additives in the bag(s) for the timepoint. Report the volume in either milliliters (mL) or grams (g). For the “at infusion” timepoint, the total volume should be the actual volume given to the recipient.
Question 44-45: Report the total nucleated cells (TNC) (Includes nucleated red and nucleated white cells)
Report Done if the TNC count was quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. Report Not done if the TNC count was not quantified at the specified timepoint.
Occasionally, cell differential results may be “corrected” in order to remove cells such as nRBCs. The CIBMTR would like to have uncorrected data submitted in these fields. Some labs report corrected cell counts, others report uncorrected cells counts. Some even report both. If your lab report does not clearly indicate whether the TNC is corrected or uncorrected, ask someone in the lab to help you determine which is correct. This will most likely be the same every time, so you would not need to check for each patient. If this information is not clearly indicated on the lab report, please ensure this is somewhere in your center SOPs. If the only value available to you is the corrected TNC, you may calculate the uncorrected TNC with the formula below. Please be sure to carefully check your math and the units reported to ensure that the information on the form is correct. To determine the uncorrected TNC count, use the following formula (Adapted from Essential Laboratory Mathematics by CW Johnson, DL Timmons, PE Hall (2003), pg 175.):
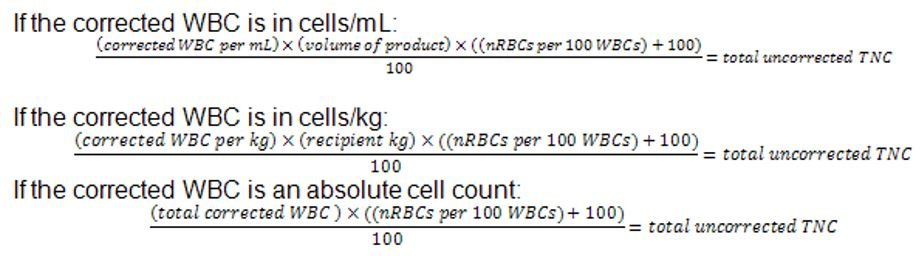
For example, if the corrected WBC is 17.96×106/mL, the product volume is 390 mL, and the nRBCs per 100 WBCs is 12.8 (using the formula above when considering cells/mL):
Questions 46-47: Viability of total nucleated cells
If the viability of the total nucleated cells was quantified, select Done and report the percentage of viable cells. If the viability was not assessed, or if it is unknown whether viability was tested, report Not done or Unknown, respectively.
If your center’s laboratory assay only measures viable cells, report the number of viable cells in Total nucleated cells, select Done for this question, and report the viability as 100%.
Questions 48-49: Method of testing cell viability
Indicate the method of testing viability.
- Flow cytometry based: 7-AAD (7-aminoactinomycin D) and Propidium iodide are compounds that can stain dead cells but will not cross the membrane of living cells. Cytometric techniques are used to calculate the percentage of viable cells in a sample.
- Trypan Blue: is a technique where the dead cells become stained when in contact with the compound, but living cells remain impermeable to the dye. Cells are counted under a microscope to determine the percentage of viable cells in a sample.
If the cell viability was tested using a different method, select *Other method( and specify the method.
Questions 50-51: Report the nucleated white blood cells
Report Done if the nucleated white blood cells (also known as leukocytes) were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the nucleated white blood cell count was not assessed, report Not done.
Questions 52-53: Report the mononuclear cells
The total mononuclear cell count includes lymphocytes and monocytes. Report Done if the mononuclear cells were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the mononuclear cell count was not assessed, report Not done.
Questions 54-55: Report the nucleated red blood cells
Report Done if the nucleated red blood cells (also known as normoblasts) were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the nucleated red blood cell count was not assessed, report Not done.
Questions 56-57: Report the CD34+ cells
Report Done if the CD34+ cells were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the CD34+ cell count was not assessed, report Not done.
Questions 58-59: Viability of CD34+ cells
If the viability of the CD34+ cells was quantified, select Done and report the percentage of viable cells. If the viability was not assessed, or if it is unknown whether viability testing was performed, report Not done or Unknown, respectively.
If your center’s laboratory assay only measures CD34+ viable cells, report the number of viable CD34+ cells in Total number of CD34+ cells, select Done for this question, and report the viability as 100%.
Questions 60-61: Method of testing cell viability
Indicate the method of testing viability.
Flow cytometry based: 7-AAD (7-aminoactinomycin D) and Propidium iodide are compounds that can stain dead cells but will not cross the membrane of living cells. Cytometric techniques are used to calculate the percentage of viable cells in a sample.
Trypan Blue is a technique where the dead cells become stained when in contact with the compound, but living cells remain impermeable to the dye. Cells are counted under a microscope to determine the percentage of viable cells in a sample.
If the cell viability was tested using a different method, select “other method” and specify the method in question 61.
Questions 62-63: Report the CD3+ cells
Report Done if the CD3+ cells were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the CD3+ cell count was not assessed, report Not done.
Questions 64-65: Viability of CD3+ cells
If the viability of the CD3+ cells was quantified, select Done and report the percentage of viable cells. If the viability was not assessed, or if it is unknown whether viability was assessed, report Not done or Unknown, respectively.
If your center’s laboratory assay only measures CD3+ viable cells, report the number of viable CD3+ cells in Total number of CD3+ cells, select Done this question, and specify the viability as 100%.
Questions 66-67: Method of testing cell viability
Indicate the method of testing viability.
Flow cytometry based: 7-AAD (7-aminoactinomycin D) and Propidium iodide are compounds that can stain dead cells but will not cross the membrane of living cells. Cytometric techniques are used to calculate the percentage of viable cells in a sample.
Trypan Blue is a technique where the dead cells become stained when in contact with the compound, but living cells remain impermeable to the dye. Cells are counted under a microscope to determine the percentage of viable cells in a sample.
If the cell viability was tested using a different method, select Other method and specify the method.
Questions 68-69: Report the CD3+CD4+ cells
Report Done if the CD3+CD4+ cells were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the CD3+CD4+ cell count was not assessed, report Not done.
Questions 70-71: Viability of CD3+CD4+ cells
If the viability of the CD3+CD4+ cells was quantified, select Done and report the percentage of viable cells. If the viability was not assessed, or if it is unknown whether viability was assessed, report Not done or Unknown, respectively.
If your center’s laboratory assay only measures CD3+CD4+ viable cells, report the number of CD3+CD4+ viable cells in the Total number of CD3+CD4 cells, select Done for this question, and report the viability as 100%.
Questions 72-73: Method of testing cell viability
Indicate the method of testing viability.
Flow cytometry based: 7-AAD (7-aminoactinomycin D) and Propidium iodide are compounds that can stain dead cells but will not cross the membrane of living cells. Cytometric techniques are used to calculate the percentage of viable cells in a sample.
Trypan Blue is a technique where the dead cells become stained when in contact with the compound, but living cells remain impermeable to the dye. Cells are counted under a microscope to determine the percentage of viable cells in a sample.
If the cell viability was tested using a different method, select Other method and specify the method.
Questions 74-75: Report the CD3+CD8+ cells
Report Done if the CD3+CD8+ cells were quantified at the specified timepoint. Report the absolute number of the cells, not cells per kg. If the CD3+CD8+ cell count was not assessed, report Not done.
Questions 76-77: Viability of CD3+ CD8+ cells
If the viability of the CD3+ CD8+ cells was quantified, select Done and report the percentage of viable cells. If the viability was not assessed, or if it is unknown whether viability was assessed, report Not done or Unknown, respectively.
If your center’s laboratory assay only measures CD3+CD8+ viable cells, report the number of CD3+CD8+ viable cells in Total number of CD3+CD8+ cells, select Done for this question, and report the viability as 100%.
Questions 78-79: Method of testing cell viability
Indicate the method of testing viability.
Flow cytometry based: 7-AAD (7-aminoactinomycin D) and Propidium iodide are compounds that can stain dead cells but will not cross the membrane of living cells. Cytometric techniques are used to calculate the percentage of viable cells in a sample.
Trypan Blue is a technique where the dead cells become stained when in contact with the compound, but living cells remain impermeable to the dye. Cells are counted under a microscope to determine the percentage of viable cells in a sample.
If the cell viability was tested using a different method, select Other method and specify the method.
Question 80: Were the colony-forming units (CFU) assessed after thawing? (cord blood units only)
CFUs have been shown to be a predictor of engraftment. Indicate whether CFUs were assessed after thawing.
Question 81: Was there growth?
If CFUs were assessed after thawing, indicate whether growth was detected.
Questions 82 – 85: Indicate which assessments were carried out (check all that apply)
Select which CFU was assessed after thawing, select all that apply.
If the total CFU-GM (granulocyte / macrophages) was quantified, select Total CFU-GM and report the total CFU-GM as documented on the laboratory report.
If the total CFU-GEMM (granulocyte / erythrocyte / monocyte / megakaryocytes) was quantified, select Total CFU-GEMM and report the total CFU-GEMM as documented on the laboratory report.
If the total BFU-E (burst forming unit – erythroid) was quantified, select Total BFU-E and report the total BFU-E as documented on the laboratory report.
Do not report CFU per dish, per bag, or per kg.
Question 86: Were any positive cultures (for bacterial or fungal infections) obtained from the product at the transplant center? (complete for all cell products)
If positive cultures were obtained, select Yes.
If positive cultures were not obtained, select No.
If cultures are pending, select Pending. If these results are reported as Pending, transplant centers will be asked to update this field once the culture results are available.
If culture results are unavailable, or if it is unknown whether culture assessments were performed, select Unknown.
The codes for “other organism, specify” (codes 198, 209, 219 and 259) should rarely be needed; check with your microbiology lab or HCT physician before using them.
Questions 87-91: Specify organism code(s)
If a single product was split into multiple bags and one or more bags are contaminated, then all bags should be considered contaminated for the purposes of reporting data to the CIBMTR.
If multiple products are infused, and only one product is contaminated, then report the infection on the Form 2006 for the product that was contaminated (i.e., the uninfected product will be reported on a separate Form 2006).
If the results were positive, select the isolated organism(s) using the pull down options in FormsNet3SM.
Section Updates:
| Question Number | Date of Change | Add/Remove/Modify | Description | Reasoning (If applicable) |
|---|---|---|---|---|
| Q41 | 7/26/2024 | Modify | Product Analysis Timepoints red warning box modified above Q41: Prior revisions of the HCT Product and Infusion (2006) Form (Revisions 1-4) have asked for product analysis values at multiple timepoints. In the new revision of the form, only the “At Infusion” timepoint is required for all product types. |
Updated for clarification for scenarios where two separate analyses are not performed prior to wash and after |
| Q41 | 7/26/2024 | Modify | Instruction for reporting timepoints for CBUs updated: For all products, the “at infusion” timepoint must be reported. The “at infusion” timepoint should only report the values for the actual product volume infused. The At Infusion timepoint should include values reflective of the product infused regardless of when the analysis occurred. Since all products are analyzed prior to cryopreservation, the At Infusion timepoint would be applicable for these cell counts. Depending on the product type and your center’s practice, viability may be assessed closer to the time of infusion. For cord blood units, |
Updated for clarification for scenarios where two separate analyses are not performed prior to wash and after |
Need more help with this?
Don’t hesitate to contact us here.

