KNOW: INVENTORY, SCREEN AND VERIFY CONTENTS
Before considering how to make improvements to a product, it is most crucial to confidently understand what is in the product.
INVENTORY
Creating a complete material ingredient inventory is a foundational step in understanding what is in a product and how to make it healthier. Conducting an inventory requires supply chain collaborations to share proprietary and confidential information between suppliers and manufacturers. Manufacturers are required to document which materials and chemicals are present in their product at a certain threshold, or level of detail. For a Declare label, manufacturers are required to inventory 100% of the ingredients present above 100ppm (.01%) in the final product.
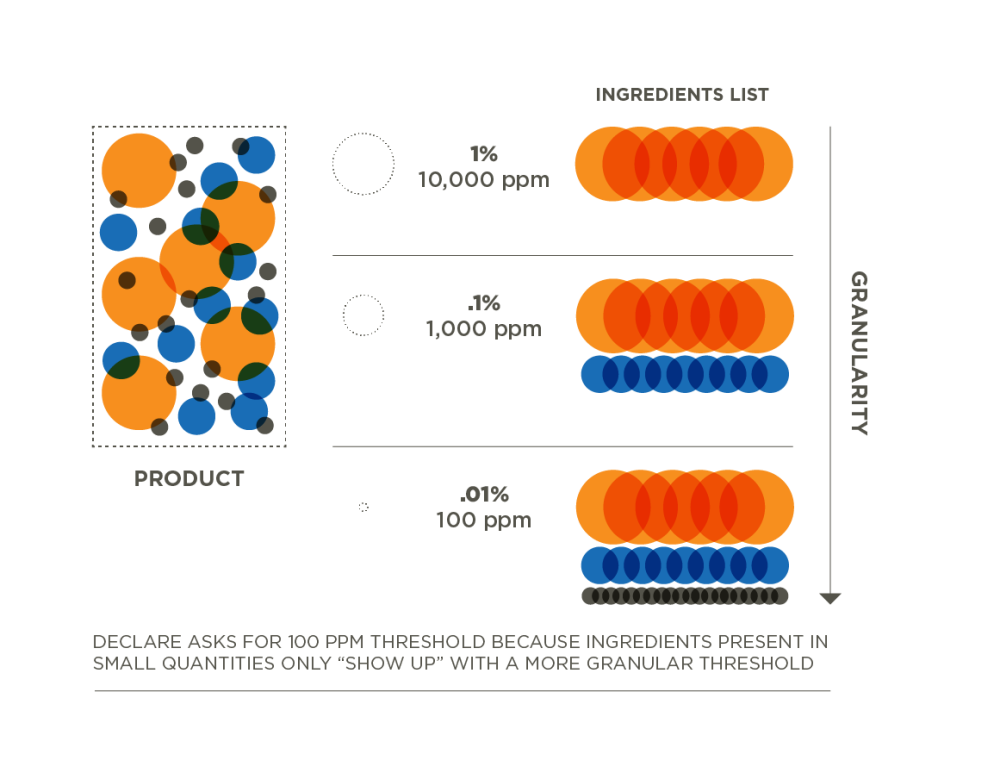
SCREEN
Once the ingredients are identified within the product to the appropriate 100ppm threshold, the next step is to screen against the Red List, by matching the unique identifier for each chemical though its Chemistry Abstract Service Registry number to identify any Red List chemicals in the product. Level of disclosure and results of screening are used to determine if a product should be marked as Red List Free, LBC Compliant or Declared. Only Red List Free and LBC Compliant labels are able to proceed with pursuit of the Living Product Challenge program. Manufacturers can work to move their product up a Declaration status through removal of Red List Ingredients or obtaining additional disclosure. For more information on Declaration statuses, please refer to the Declare Manufacturer’s Guide.
VERIFY
The Living Product Challenge requires that the material ingredient composition of all Living Products be verified by an ILFI-approved independent 3rd Party Verifier to ensure compliance with program requirements. The Declare program has a proprietary ingredients exception that allows manufacturers to withhold up to 1% of the product inventory present at or above 100ppm. However, the assessor must be able to confirm that the undisclosed 1% is indeed free of Red List ingredients, even if the information is withheld on their label.
Verification provides an unbiased evaluation of accuracy according to the Declare and/or Living Product Challenge Standard, adding rigor to the process and enhances confidence in company claims and communication with consumers. Verification also means that companies can confidently take next steps to look more deeply at the materials and any hazard implications, knowing that they won’t be missing information.
DISCLOSE: PUBLIC ACCOUNTABILITY THROUGH TRANSPARENCY
Once the material ingredient inventory has been completed and verified, public ingredient disclosure using a 3rd Party Verified (3PV) Declare label is a requirement of International Living Future Institute’s Living Product Challenge program. Declare labels are required to be available on the Declare Database, but can also be made public on the manufacturer’s website.
ASSESS: BEYOND LIST-BASED APPROACHES
The resulting Third Party Verified (3PV) Declare label can then become the basis for a deeper evaluation of the ingredients in the product. Products pursuing Living Product Challenge I09 Transparent Material Health Imperative must undergo a more rigorous assessment for carcinogenic, mutagenic, and reprotoxics (CMRs), as well as Persistent, Bioaccumulative, Toxic (PBT) chemicals.
The LPC Material Health Assessment goes beyond screening of chemicals to provide analytical information about human and environmental health hazards of ingredients across the value chain where hazard may not be known or yet clearly identified. Each assessment uses scientific literature and modeling tools to determine how a given chemical performs against multiple hazard endpoints (i.e. neurotoxicity, carcinogenicity), as well as environmental toxicity and fate of the chemical.
This deeper evaluation of chemicals with hazards that are as of yet unknown allows for greater scrutiny of the materials in a product and allows manufacturers to make comparisons between substitutes to determine how they perform along those same indicators. This is especially useful for identifying alternative ingredients that do not have unintended consequences (thereby avoiding regrettable substitutions), and truly have a more positive material health benefit.
OPTIMIZE: LEVERAGING KNOWLEDGE FOR SAFER MATERIALS
Once a manufacturer has determined the detailed composition of its product, has screened these chemicals against the Red List, verified and disclosed the ingredient information, and has gone deeper to individually assess the chemicals and materials for material health, the last step is to thoroughly evaluate and act on that information.
If the Material Health Assessor determines that no CMRs or PBTs are present in the product, the product is considered optimized.
If there are CMRs or PBTs present, the Assessor will conduct a risk and exposure analysis, to look at the exposure pathways and determine whether there is risk of exposure to human health and the environment. If a risk of exposure exists to the final manufacturer, the installer or the end user of the product, the assessor must then determine whether that risk can be reasonably mitigated. If the risk cannot be mitigated, and the assessor determines that the product is not in compliance with Imperative requirements, the manufacturer will be required to make product changes in order to bring the product into compliance.



