Standard Language:
Living Products are safe for human exposure. Going beyond the avoidance of known problematic chemicals, manufacturers intentionally select chemicals and materials proven safe throughout their life cycle.
Manufacturers must conduct a hazard assessment for all intentionally-added chemical substances in the product using an Institute-approved toxicologist. The analysis must demonstrate that the product does not create a present a reasonable risk of exposure to Carcinogens, Mutagens and Reproductive toxicants or Persistent Bioaccumulative Toxins for the manufacturer, installer or end-user. To limit assessment costs, manufacturers are allowed five percent of the product by weight to remain unassessed for initial certification; however, the product must be 100% assessed by the time of recertification. Exceptions in Imperative 09 may allow for more gradual progress to achieve 100% assessed.
The results of the Living Product Challenge Material Health Report must be transparent and publicly available.
Methodology Overview
Based on the inventory and backup documentation gathered as part of the achievement of I08, the manufacturer will work with an ILFI-approved Material Health Assessor (MHA) to achieve the Transparent Material Health Imperative. The Third Party Verified Declare label and associated documentation is used by an ILFI-approved Material Health Assessor to conduct an assessment of the ingredients. The objective of this Imperative is to eliminate any chemicals and ingredients identified as Carcinogens, Mutagens and Reprotoxins (CMRs) or Persistent Bioaccumulative Toxics (PBTs) that have been determined to be a risk to the final manufacturer, the installer or the end user of the product.
During the initial certification period (the time until the product is certified), the contracted MHA must assess a minimum of 95% of the product ingredients present above the 100ppm threshold by weight. The manufacturer then has 3 years (until recertification) to assess the remaining 5% of the product to achieve full assessment of all chemicals present above 100ppm in the final product. This allows the manufacturer to spread out assessment costs, while requiring improvement of data and materials over time.
Any Third Party Verified (3PV) Declare labels not claiming the proprietary ingredients exception can be used as documentation for the Transparent Material Health Imperative since external verification of the information has already been completed. A 3PV Declare label using the proprietary ingredients exception must produce ingredient and CASRN information for any proprietary ingredients so that the MHA can use this in their evaluation. If polymers are present in the formulation, some additional data may be required, and is noted below in the outlined methodology.
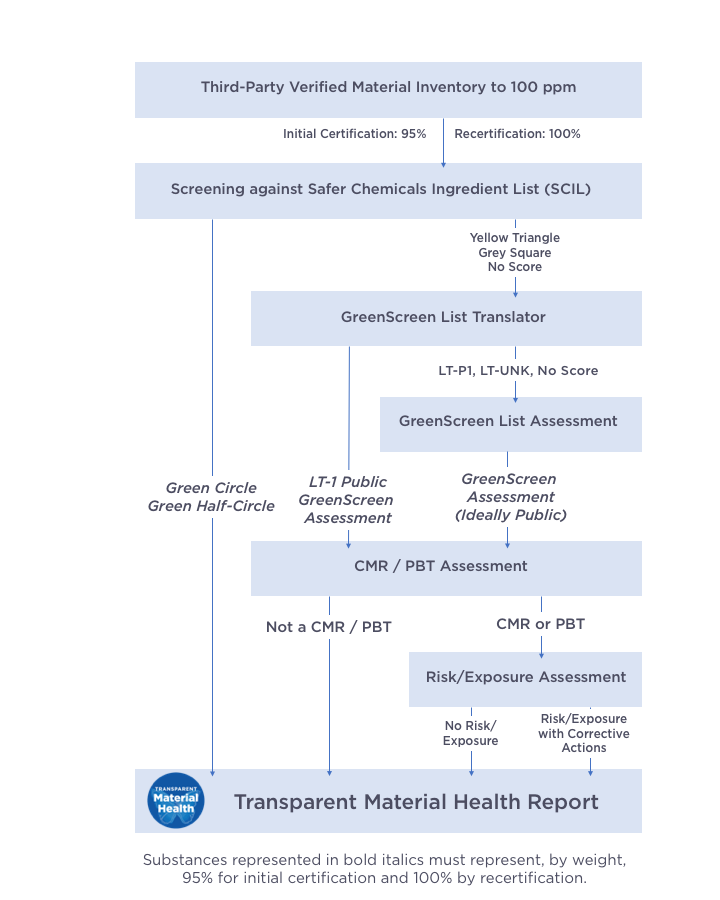
The following flowchart provides an overview of the assessment process conducted by Material Health Assessors:
Transparent Material Health Review:
1. Inventory Review
Material Health Assessors will review the verified product inventory through the Declare program in the ToxNot platform, and determine if any additional information is necessary for the assessment, such as additional polymer information, use and installation instructions for exposure assessments, or ingredient-specific information for products without CAS numbers.
2. Hazard Assessment
Assessors determine the material health hazards of the product content using three tools: the Safer Chemicals Ingredient List, the GreenScreen List Translator, and the GreenScreen for Safer Chemicals methodology:
- Safer Chemicals Ingredient List (SCIL) Screening: the SCIL is a list of chemical ingredients, arranged by functional-use class, evaluated by the US EPA Safer Choice Program, which can help manufacturers quickly and economically find safer chemical alternatives to ingredients. These ingredients have been determined to be safer than traditional chemical ingredients.
- Ingredients with a designation of green circle, half green circle, and yellow triangle do not require additional assessment.
- GreenScreen List Translator Screening: compares the ingredient CASRN with authoritative hazard lists to identify any known hazards. GreenScreen List Translator screening generally results in a designation of LT-1, LT-P1 or LT-UNK.
- LT-1 chemicals require a summary by the assessor, including a CMR/PBT assessment, but do not require a full GreenScreen assessment to be performed.
- Any chemical with an existing, publicly available GreenScreen Benchmark Score does not require additional assessment.
- GreenScreen for Safer Chemicals Hazard Assessment: goes beyond screening against the Red List or using the GreenScreen List Translator by looking to scientific literature to determine where in the life cycle a chemical presents certain hazards, to what degree, and which pathways (dermal, inhalation, etc.) present this impact.
- The assessment process results in a final hazard assessment for each chemical in terms of Benchmark scores 1-4, with one (1) being the lowest and targeted for elimination, and four (4) being the safest. Chemical ingredients are assessed based on 18 hazard endpoints.
- GreenScreen Assessment Benchmark scores (BM-1 to BM-4) need to be made publicly available so that at least 75% by weight of the product’s hazard assessments are publicly available, calculated by summing the percent weight of the product that meets the SCIL requirements in part (a), LT-1 scores, and publicly available GreenScreen Assessments. Upon recertification, the amount required to be publicly available needs to be 95% of the weight of the product.
- If the product can achieve the required public disclosure amount utilizing the screening requirements of parts (a) and (b), at least one publicly available GreenScreen Assessment shall be performed (unless all assessment information for the product inventory is already publicly available).
Utilizing the results of the hazard assessment outlined above, any chemical that does not score a Green Circle or Green Half-Circle on the SCIL will be assessed using GreenScreen methodology to determine if it is a CMR or PBT, to be defined as:
- Carcinogen – GreenScreen endpoint C is [H or vH]
- Mutagen – GreenScreen endpoint M is [H or vH]
- Reproductive Toxin – GreenScreen endpoint R is [H or vH]
- Persistent, Bioaccumulative, and Toxic Chemicals – GreenScreen endpoints meet all of the following:
- [H or vH] for P
- [H or vH] for B
- [vH] Toxicity (Ecotox or Group II) or [H or vH] Toxicity (Group I or II*)
- H or vH points to hazard level of the endpoint; H indicating High and vH indicating Very High.
3. Risk + Exposure
For any hazard assessments that result in a CMR or PBT designation as outlined above, the Material Health Assessor will then conduct a Risk and Exposure review of that chemical. This evaluation will determine whether reasonable risk of exposure exists to the final manufacturer, the installer or the user of the product. In some cases, the review will determine that a CMR or PBT in a product has no risk of exposure to the user, and therefore does not need to be removed as per LPC requirements. If any chemicals still remain with the designation of CMR or PBT after the review, then product changes must be implemented.
4. LPC Transparent Material Health Report + Corrective Actions
Once the review is complete, the Material Health Assessor produces a Transparent Material Health Report to be made public along with the product’s LPC label and case study. This Report shall contain a summary of the hazard assessment for each chemical ingredient present in the product’s formulation, including the results from each step in the Hazard Assessment methodology above. Additionally, the report also provides the risk and exposure reviews for any chemical ingredient that was assessed as a CMR or PBT.
The report summarizes the findings and provides the manufacturer with a final determination (achieved or denied) and a list of Corrective Actions that the MHA determines need to be addressed. ILFI reviews the report and determines if any actions are outside the scope of the LPC review and therefore do not need to be completed by the manufacturer. The manufacturer is then provided a timeline in which those actions need to be addressed and completed before recertification in order to maintain achievement of the Imperative.
If a product fails the assessment, the manufacturer is required to make product or process changes to bring the product into compliance as per the report recommendations before the Imperative will be awarded.
The results of this report should be made publicly available so that any risk and exposure determinations can be evaluated publicly. The report shall also provide a one page product overview, using the table below as an example.
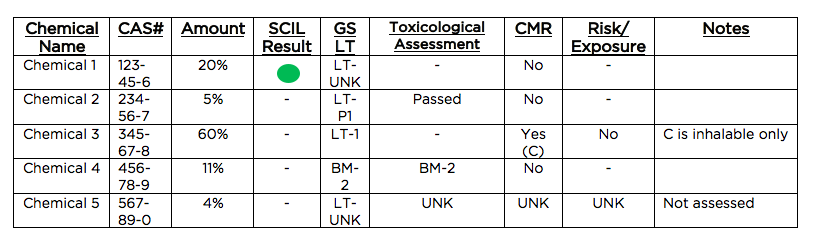
The example above indicates 96% of the product has been assessed, meaning it would qualify for the initial certification. The benchmark scores indicate that 71% of the assessments are publicly available.
5. Temporary Exceptions
The Institute may, on a case by case basis, create temporary exceptions if it is demonstrated that there are legitimate industry-wide market limitations, performance concerns for a specific application with no alternatives, or if a manufacturer provides a reasonable timeline for removing a particular chemical. The manufacturer is then provided a timeline in which those actions need to be addressed and completed. These temporary exceptions are published in the LPC 2.0 Guide and are intended to be removed over time.
6. Improvement Actions
A truly healthy product goes beyond being CMR and PBT (GreenScreen Benchmark 1) risk free. It is completely safe for humans and the environment. Therefore, the Material Health Assessor should include in the report a list of Improvement Actions designed to provide the manufacturer with a pathway for making the product as safe as possible. These actions do not prevent the product from achieving the Imperative during certification. ILFI reviews the report and determines which of the improvement actions should be completed by the manufacturer, based on their relevance to the review. The manufacturer is then provided a timeline in which those actions need to be addressed and completed before recertification.