For reproducible and comprehensive immune profiling analysis, it is essential to use a technology that provides the highest efficiency for amplification of both high- and low-abundant clonotypes, and accomplishes this with a small amount of input template (e.g., 10-100 ng for RNA or 0.5-5 ug for DNA). Currently, multiplex PCR and 5’-RACE (SMART) are two commonly used targeted CDR3 amplification technologies for immune receptor repertoire analysis [1-5].
Recent benchmark studies of commercially available immune receptor profiling assays show that 5’ RACE (SMART) for RNA and multiplex PCR for gDNA have the best sensitivity for comprehensive repertoire analysis [8,9]. However, Cellecta has developed the novel, ultra-sensitive, multiplex RT-PCR DriverMap AIR assay that detects three-fold more clonotypes than the conventional SMART-based assay with the same input amount (50 ng of PBMC RNA), as shown in Fig. 8. Both technologies identify a similar set of the top abundant TCR clonotypes with less reproducible detection of medium to low-abundant clonotypes. Importantly, sensitivity, specificity, and reproducibility are significantly higher with the DriverMap AIR RNA multiplex technology when compared to SMART technology (Fig. 9).


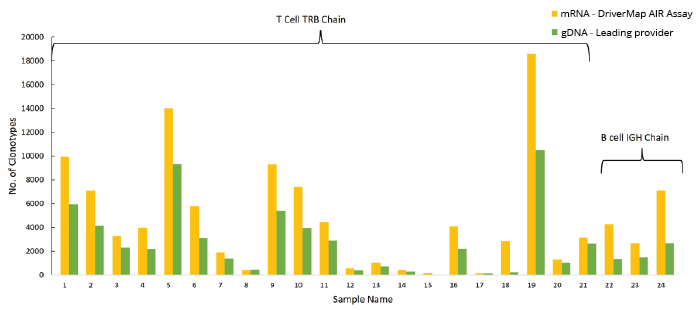
We also compared the performance of the AIR RNA assay with a leading commercial gDNA-based multiplex immune receptor profiling assay. DNA and RNA were extracted from sorted T and B cells from metastatic tumor samples. Profiling studies of both immune receptors were then conducted in parallel [20]. Fig. 10 shows that the AIR RNA assay identifies 1.5 to 2-fold more clonotypes than the leading gDNA-based multiplex PCR assay.
Need more help with this?
Contact Us

