In this step, complementary DNA (cDNA) is generated from total RNA. All reactions must be set up in 96-well plate format (standard, or separate tubes/strips) using approximately the same adjusted starting amount of RNA across all reactions.
For best results, we suggest starting with 10-50 ng of high quality total RNA (or 50-500 ng of FFPE RNA). Reliable results with as little as 10 pg of high quality RNA are possible for most genes, but some transcripts expressed at low levels may not be accurately measurable. Starting RNA sample volumes must be equal or less than 6 µl.
If you used the DirectCell™ Oligo-dT Capture Protocol, the mRNA captured from cell lysates in oligo-dT plates and resuspended in 6 µl can be used directly for cDNA Synthesis. Go directly to Step 2 in the procedure below, and then add the RT Master Mix to the TurboCapture oligo-dT plate (QIAGEN).
For high quality RNA, do not use more than 100 ng in the Reverse Transcription (RT) reaction. Too much RNA can cause non-linear target amplification.
We highly recommend reserving at least one well for the Positive Control RNA at a concentration as close as possible to that of your experimental samples. If running several experiments, it can be useful to include the same amount of Positive Control RNA with each sample run. This will help with troubleshooting, data analysis, and normalization across different sample runs.
If processing fewer than 96 reactions, fill empty wells with water to minimize evaporation. The figures below show example reaction arrangements for 22 or 94 experimental samples plus the two controls for the 24-reaction and 96-reaction kits, respectively.
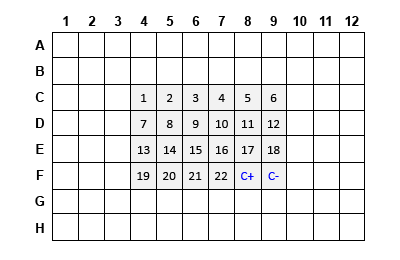
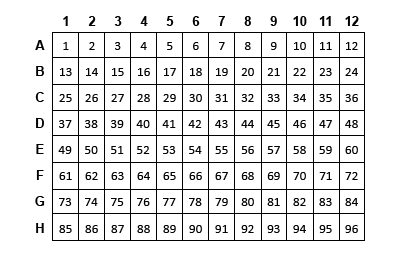
- Aliquot an equal amount of each starting total RNA in the wells of a 96-well plate and adjust the volume of each sample to 6 µl with PCR-Grade Water as shown in the table.
Component Volume per sample, µl Total RNA (0.01 – 50 ng) 1 – 6* PCR-Grade Water, to 6 µl final volume 0 – 5 Total 6
- Prepare an RT Master Mix as described below for all samples and controls plus 5% extra volume of all components as a safeguard against pipetting error:
RT Master Mix Component Volume per sample, µl RT-EXT Buffer 2 dNTP Mix 0.5 Random Primers 1 PCR-Grade Water 1 Reverse Transcriptase 0.5 Total 5
- Add 5 µl of RT Master Mix to each well, and mix contents by pipetting 3 times. Seal the plate with adhesive film, and spin down to collect droplets. Load the plate in the thermal cycler, and run the following program to synthesize cDNA:
Temperature Time 50°C 30 min 95°C 5 min 4°C ∞
Need more help with this?
Contact Us

