In this step, the yield of products from the Index Primer PCR Reaction—the Amplified Indexed Libraries of transcripts from each sample—are analyzed (adjusted if necessary), measured, and then pooled in equimolar amounts for sequencing.
QC and Quantify Amplified Indexed Libraries
- Analyze the Amplified Indexed Libraries using one of the following methods:
- Standard Method: Separate 5 µl of Amplified Indexed Libraries on a 3% agarose-TAE gel and analyze the size distribution of NGS probes by UV transilluminator. See below for the expected results of amplified libraries generated from experimental and positive control RNA samples. For the hGW19K assay, the smear with several bright bands is usually in the 180-450 bp range.
- Alternative Method: Analyze 1 µl of each of the Amplified Indexed Libraries on either an Agilent Bioanalyzer with the Agilent High Sensitivity DNA Kit (Cat.# 5067-4626) or Fragment Analyzer using the High Sensitivity NGS Analysis Kit (Cat.# DNF-473-1000) using the manufacturer’s protocol.
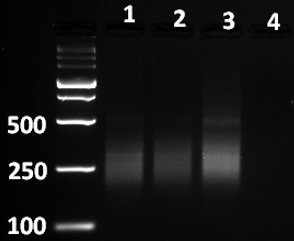
- Analyze yields of the Amplified Indexed Libraries. If amplified cDNA products are generated from samples with similar content of mRNA, the yield should all be roughly the same for all experimental samples within 2-3-fold levels. A negative control sample should not generate any significant yield of amplified products. If some samples show significantly lower yield (e.g., >5-10-fold) of amplification products it could be an indication of differences in the mRNA content or quality of RNA used in the assay. If several or all the experimental RNA samples have significantly lower yield (not detected or very low intensity in comparison with positive control RNA), you can rerun these experimental RNA samples in the thermal cycler for 2-5 additional cycles. After cycling, quantify the products again relative to the Positive Control RNA. Adjustment for small differences (e.g. 2-5-fold) in the yield of amplified products between samples will be made when the amplified Indexed Libraries are quantitated (in Bioanalyzer) and combined for the sequencing step following this QC step.
Remove Excess PCR Primers and Combine Samples
- Remove excess primers from the completed PCR reactions by adding 1 µl of Primer Removal Enzyme to each of the Amplified Indexed Libraries and the Negative Control sample, then incubate at 37°C for 30 minutes. Do not inactivate the Primer Removal Enzyme by incubating the reaction at a high temperature, as it will melt the double-stranded PCR products and complicate the analysis of PCR products in the Bioanalyzer.
- To ensure accurate quantification for sequencing, especially for samples with low yield of amplified products, you could consider repeating the quantification procedure of the Amplified Indexed Libraries. Quantifying the PCR products after the removal of PCR primers is more accurate than quantifying before the primer removal clean-up step. The preferred method is to analyze 2 µl of each of the Amplified Indexed Libraries using either an Agilent Bioanalyzer with the Agilent High Sensitivity DNA Kit (Cat.# 5067-4626) or Fragment Analyzer using the High Sensitivity NGS Analysis Kit (Cat.# DNF-473-1000) using the manufacturer’s protocol.
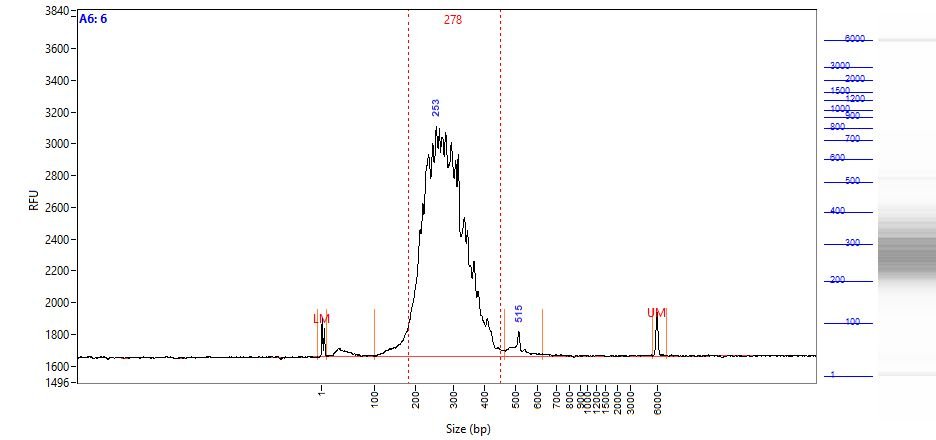
- Quantitate the yield of Amplified Indexed Libraries using smear analysis of amplified products in the region of 180-450bp (see Fig.4). Use the yield assessment of the Amplified Indexed Libraries as a basis to combine equimolar amounts of each of the Amplified Index Libraries into a single pool for NGS. For example, if the yield of Library 1 is twice that of Library 2, then mix 5 µl of Library 1 with 10 µl of Library 2. To minimize sample-to-sample sequencing variations, combine and load all experimental samples onto one flow cell.
The DriverMap™ EXP kit generates approximately 10,000-15,000 amplified products, so MiSeq, NextSeq, and NovaSeq Illumina instruments can be used for NGS. We recommend using the NextSeq 2000 with the newest XLeap-SBS assay chemistry which provides the most cost-effective solution to sequence 10-1,000 samples in a single flow cell. MiSeq could be used to sequence a small number (5-10) of samples. The NovaSeq X instrument can be used for sequencing a large number of samples such as 384 (four 96 well plates) by combining with other NGS samples in one flow cell (with a minimum capacity of 8B reads per flow cell). Please, refer to the table below for guidelines on how many samples may be combined for different instruments (MiSeq and NextSeq) and read depths. Generally, you should aim for approximately 5M reads per sample for regular and 10M reads for high-sensitivity NGS. For Illumina instruments, hGW19K assays require the use of 100-cycle or 150-cycle Paired-End reagent kits.
| Instrument | Reads per flow cell | Number of samples for multiplexing per flow cell |
Reads per sample |
|---|---|---|---|
| MiSeq Series | 50 million (v3) | 10 | 5M |
| NextSeq 500/550 | 120 million (Mid Output) | 24 | 5M |
| NextSeq 500/550 | 400 million (High Output) | 80 | 5M |
| NextSeq 2000 | 100 million (P1 flow cell) | 20 | 5M |
| NextSeq 2000 | 400 million (P2 flow cell) | 80 | 5M |
| NextSeq 2000 | 1.2 billion (P3 flow cell) | 150-250 | 5M-10M |
| NextSeq 2000 | 1.8 billion (P4 flow cell) | 192-384 | 5M-10M |
Need more help with this?
Contact Us

